Abbott's FreeStyle® Libre 2 iCGM Cleared in U.S. for Adults and Children with Diabetes, Achieving Highest Level of Accuracy and Performance Standards - Jun 15, 2020
Abbott (NYSE: ABT), the worldwide leader in continuous glucose monitoring (CGM), announced today the U.S. Food and Drug Administration (FDA) cleared its next-generation FreeStyle® Libre 2

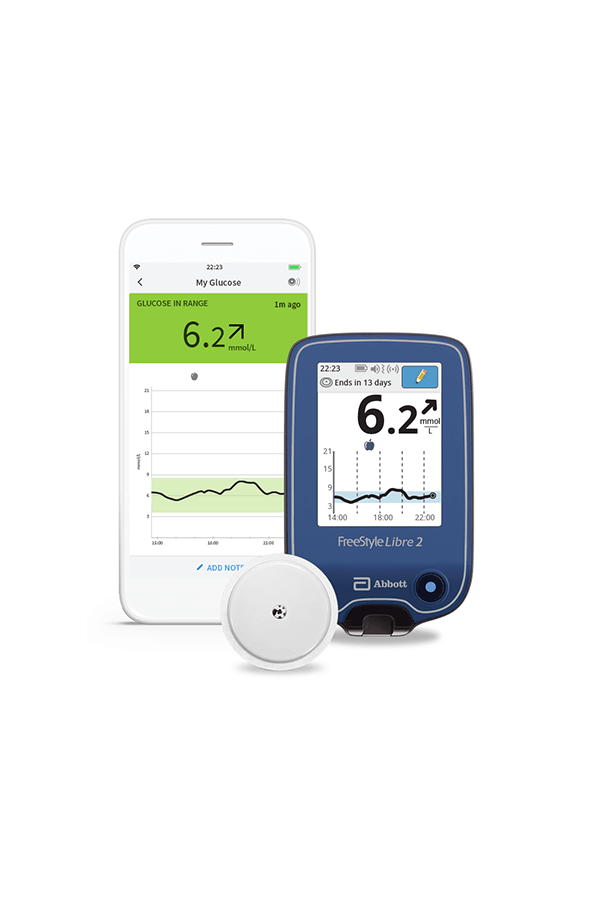
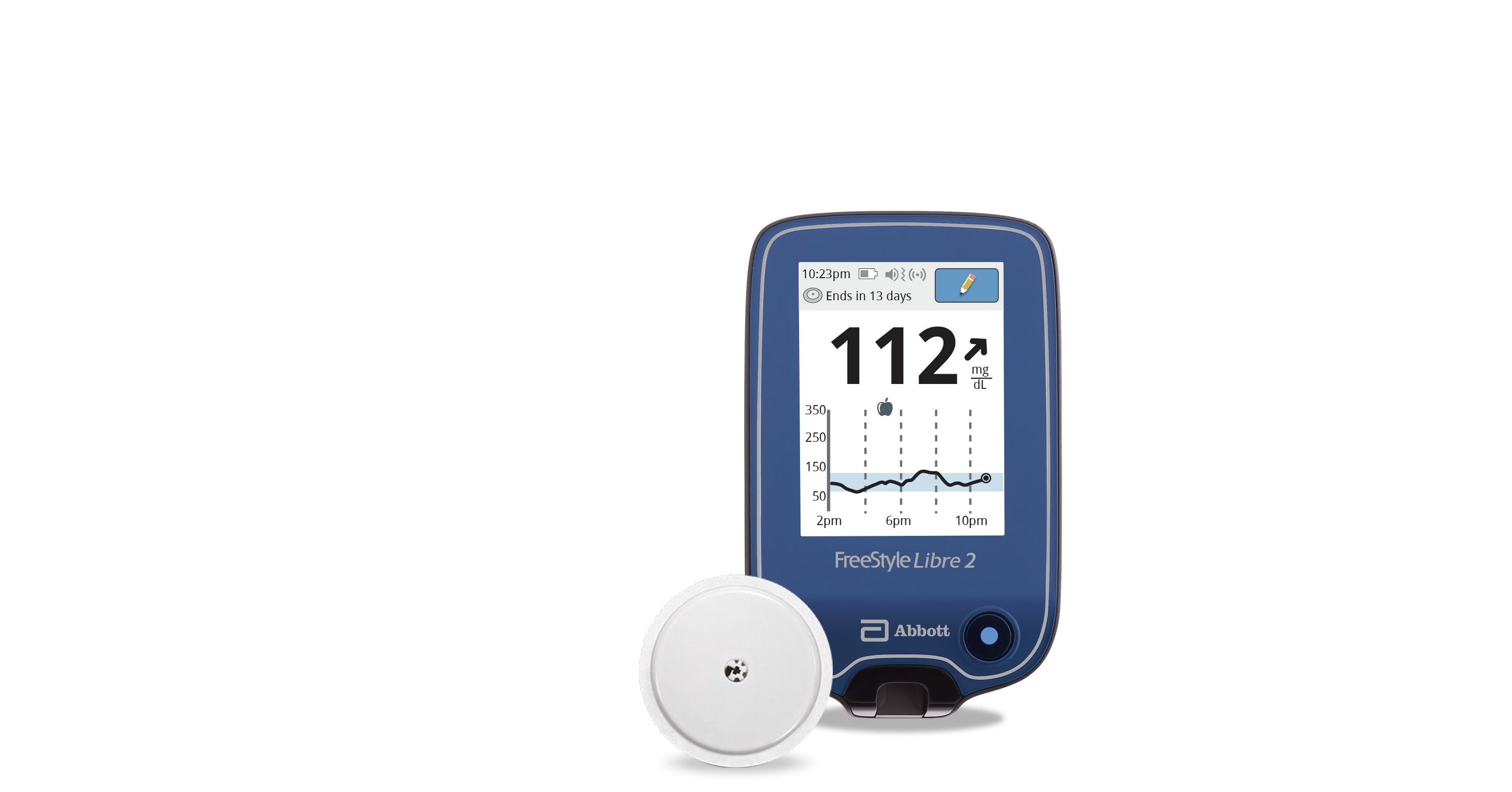
FreeStyle Libre 2 Continuous Glucose Monitor

FreeStyle Libre 2 System (CGM)

FreeStyle Libre Continuous Glucose Monitoring
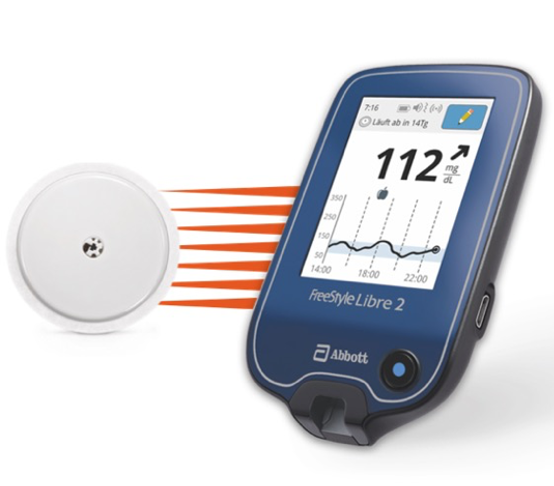
FreeStyle Libre 2 Cleared by FDA as iCGM
FreeStyle Libre 2 System (CGM)

Abbott's Freestyle Libre system becomes first CGM to be FDA cleared for use without fingersticks

Improving Equitable Access to Continuous Glucose Monitors for Alabama's Children with Type 1 Diabetes: A Quality Improvement Project
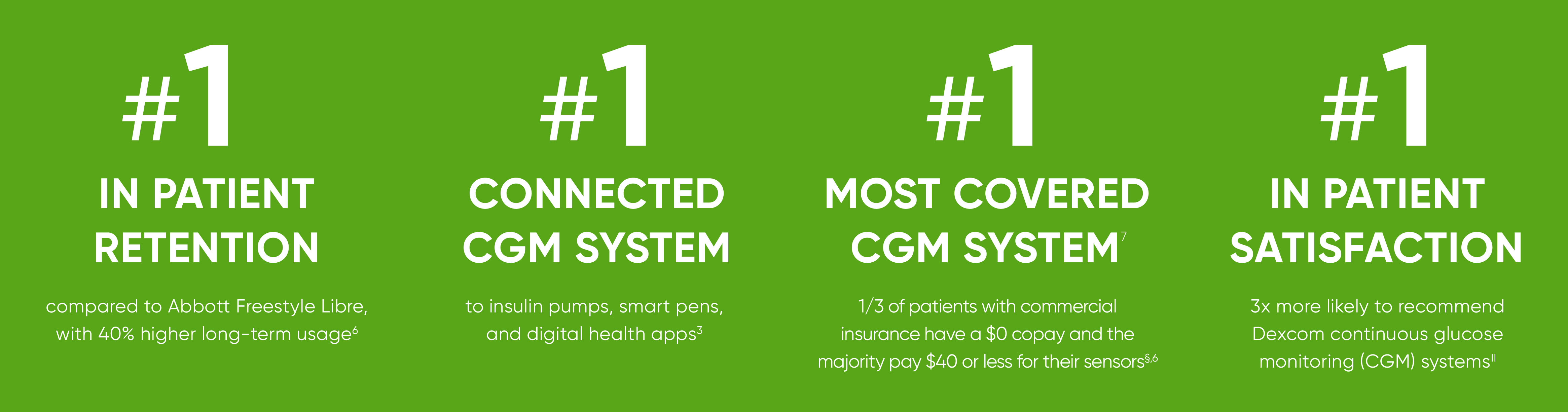
Dexcom G6 CGM System for Personal Use

Freestyle Libre 2 - Continuous Glucose Monitoring System
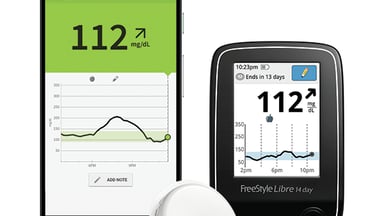
ADA: Real-world data show lower A1c levels with Abbott's FreeStyle Libre CGM in Type 2 diabetes

Comparison of Point Accuracy Between Two Widely Used Continuous Glucose Monitoring Systems - Kevin Hanson, Mark Kipnes, Hien Tran, 2024

FDA Approves FreeStyle Libre 2 iCGM System for Patients With Diabetes